Pharmacogenomics: The Exciting Science of Personalized Medicine
Nov 01, 2017
23535 Views
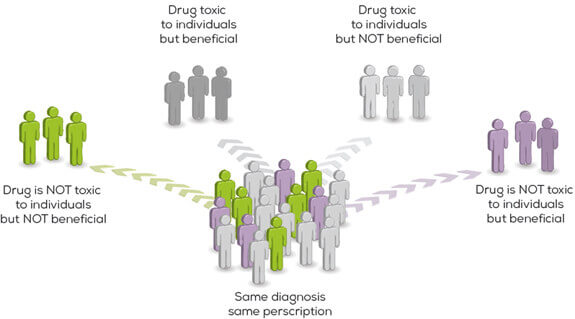
We are unique individuals at the genetic level. We all respond to our environments differently. Same is the case with the medicines we take. Adverse drug reactions are the fourth largest cause of deaths ahead of diabetes, accidents (including automobile deaths) and AIDS! And yet the simple understanding that every medicine will act differently in every individual can save so many lives. This is what the exciting science of Pharmacogenomics (PGx) aims to achieve.
Pharmacogenetics or Pharmacogenomics (PGx), as the name indicates, is a combination of pharmacology (study of uses, effects and mode of action of drugs) and genomics (study of structure and function of genes). It studies how a person’s inherited genes (or gene variants) influence his or her response to drugs. The main aim of Pharmacogenomics is to administer the correct drug to the correct person at the correct dose, thus reducing the side-effects.
Benefits of Pharmacogenomics
The human body has different genes that are responsible for physical characteristics such as eye colour, skin colour and type of hair on the head. They are also responsible for the chemical and biological responses against a particular drug. Until pharmacogenomic testing was introduced, drug development was carried out assuming all drugs act similarly in all humans. But genomics indicated otherwise. Even if we share 99.9% of our DNA, the 0.1% has answers for the way our bodies react to drugs. This difference means a drug that may benefit one person may be harmful to another. Pharmacogenomics attempts to offer a solution to this and confers the following benefits.
- Correct and safer drugs from the beginning: By studying the gene sequence of a particular person, variations in genes can be identified that govern response to drugs. Thus, your doctor will be able to predict how your body will react to a particular drug type and hence select the best drug and its dose, tailor-made, just for you!
- Apart from the genetic indications, doctors will consider other factors like your age, lifestyle, habits, concurrent medications and diseases and your overall health while deciding on the medicines and their dosage.
- Time- and cost-effective: Pharmacogenomic testing will also reduce the money and time required to find the best suited drug, usually decided by the trial-and error method. It will limit the drug choices and hence the time patients are on medication. Your DNA does not change with time and hence you will need a pharmacogenomic testing only once for a particular drug. However, if you have to take another medication, a different pharmacogenomic test, specific to that particular drug will have to be carried out. Each drug is associated with a separate pharmacogenomics test.
- Proper doses: As of now, doses are decided on the basis of the patient’s age and weight. This practice can be replaced with deciding the dosage based on the genetic make-up, thus reducing the chances of overdose.
All these benefits conferred by Pharmacogenomics testing only point towards a better and safer patient healthcare routine.
Challenges
A commentary appeared in Nature Biotechnology in 1998 about the growing need and importance of pharmacogenomics [1]. The vision that the discovery of genetic variances that affect drug action will lead to development of targeted and customized diagnostic procedures and therapeutic products and will enable drugs to be selectively prescribed to patients depending upon their efficacy and safety was surreal and almost fantastic.
It’s been close to two decades since the importance and need of this new science was propounded. The last two decades have seen exciting studies and research that has led to pharmacogenomics grow as a science and as a tool with great potential.
Pharmacogenomics is an investment whose cost-benefit ratio clearly outperforms the economic burden of conventional medicine practice. Not only does it benefit individual patients with alleviating the stress of multiple medications and their effects on the body, it also reduces the economic burden of treating patients.
There still are certain ground realities that we have to contend with but the science is infallible and holds a lot of promise. Firstly, since it is relatively new, pharmacogenomic testing can be very expensive. Also, more than one tests are required if you are on multiple medications. Unfortunately, pharmacogenomic tests are not available for all drugs. Thus, it is up to the doctor to decide whether or not you need pharmacogenomic testing. Another question that has to be tackled is limited drug alternatives. There may be a small number of drugs approved for treatment. Hence, if through testing it comes to light that a patient is unfit to use these drugs, there may be no medicines for treatment.
Where is the proof?
A recent study completed by the University of Utah concluded that use of the principles of pharmacogenomics reduced hospitalizations by 39% and ER visits by 79% in elderly polypharmacy patients, in 4 months. Risk of death was found to be reduced by an impressive 85% and a per capita cost savings of $4,382 in only 60 days, was calculated [2]. If this study’s numbers could be reproduced across the entire population spectrum, it could result in countless lives saved, not to mention the massive cost savings.
Pharmacogenomic testing in cases of HIV have revealed that some patients have an allergic reaction towards the drug Abacavir. This allergic reaction in HIV patients can be fatal. Now, the genes that control these allergic responses include a gene variant HLA-B*5701. Thus, screening of HIV patients for this particular variant has enabled doctors to identify patients with this gene and thus administer an alternative antiretroviral drug [3].
Another example of successful pharmacogenomic testing is in the case of rheumatoid arthritis. Azathioprine, a drug that is administered to organ transplant patients and also to treat autoimmune diseases is prescribed only after pharmacogenomic testing for the gene variant TPMT. Azathioprine has to be converted into its active form, which is done by the TPMT enzyme. In individuals with a TPMT gene variant, Azathioprine remains in its inactive form, accumulating in the bone marrow, killing the developing blood cells and thus making the patient vulnerable to infections. Pharmacogenomic testing has allowed healthcare providers to screen patients for this gene variant and determine if Azathioprine can be effective for them [4].
What should you ask your doctor?
Before opting for pharmacogenomic testing voluntarily or going ahead on your doctor’s advice, do your own reading and understand how your genes will respond to the drugs. Some questions that you can consider asking are:
- Which treatment or combination of treatments will I be given?
- What are my therapy options?
- How will my body react to the particular treatment? Can this be predicted?
- Are there any side-effects to these treatments? What are they?
It is advisable to gather as much information and clarity on the subject as you can before you go ahead with pharmacogenomics testing.
Don’t assume; Assess
Gone are the days when one could only assume a drug works for him/her, maybe because a prior experience with the same was a fruitful one, or maybe because everyone in his/her family took that drug and didn’t report any adverse reactions. With the advent of sequencing technologies, and the ever-expanding field of genetic research, you can get your pharmacogenomic profile done to assess your response for ~100 FDA approved drugs, with a simple saliva swab! Get insights on drug efficacy and the risk of adverse reactions, based on your genetic profile.
Discover “The-Why” behind your response to “Dawai” (sic).
Welcome to the world of precision medicine with MedicaMap.
Check out our ultimate pharmacogenomic offering – MedicaMap which reports your pharmacogenomic profile for almost 100 FDA approved drugs, spread over 20+ drug classes. Common cold to Cancer – we have you covered. Visit the product page by clicking here to know more, and don’t forget to download the technical document.
Conclusion
We are all too familiar with adverse drug events as being among the top causes of deaths. Now, we also know that genetic variations lead to adverse drug reactions. Wouldn’t it be safe to assume that using pharmacogenomics to reduce adverse drug incidences to save patient lives should be a key consideration in today’s medical practice? It is no secret that all clinicians strive relentlessly to improve the quality of life for their patients. The knowledge about how a specific drug is being metabolized by a patient and what are the cumulative effects of multiple medications, will greatly aid in these efforts.
References:
- David Housman, Fred D. Ledley. Why Pharmacogenomics? Why Now? Nature Biotechnology 16, 2 – 3 (1998)
- http://youscript.com/new-precision-medicine-study-confirms-dramatic-reduction-in-readmissions-and-er-visits-in-polypharmacy-home-health-care-patients/
- Ma JD et al. HLA-B*5701 testing to predict abacavir hypersensitivity. PLoS Curr. 2010; 7(2)
- Dean L. Azathioprine Therapy and TPMT Genotype. Medical Genetics Summary 2012
