Why Genetic Testing Matters for Neurological Conditions
Feb 12, 2024
3178 Views
Neurological disorders present intricate challenges, often veiled in mystery and uncertainty. The advent of genetic testing has transformed the field, offering a powerful tool to unravel the complex genetic landscapes that underlie these disorders.
Public health challenges reveal that out of one billion people affected by neurological disorders worldwide, 50 million suffer from epilepsy, and 24 million from Alzheimer‘s and other dementias.
The Importance of Genetic Testing in Neurological Conditions:
The connection between genetic factors and neurological disorders is widely recognized. Advances in molecular genetic technology have significantly progressed, leading to the identification of new diseases and their causative genes. An impressive 80% of identified genes are actively expressed in the brain, and 40% of all genetic disorders involve the nervous system to varying degrees, according to the OMIM database 2
Genetic heterogeneity linked with a phenotype and phenotypic pleiotropy associated with a genotype can introduce complexities in clinical presentations. Therefore, a thorough understanding of the intricacies of genetic testing is imperative for clinicians. This familiarity equips them to navigate the challenges presented by genetic variations in neurological disorders, facilitating more accurate diagnoses and tailored treatment approaches.
Various genetic testing methodologies are available for identifying mutations and diagnosing conditions, including Next Generation Sequencing, Sanger sequencing, chromosomal microarray, targeted gene sequencing, etc. The decision on which test to proceed with depends on the type of chromosomal and gene abnormalities associated with a disease.
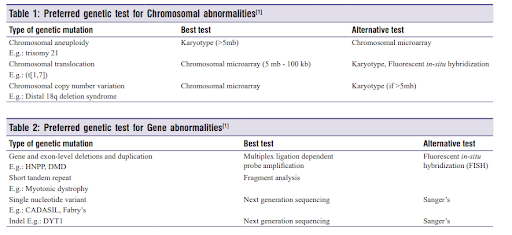

A real-life case study from MapmyGenome illustrates how genetic testing and exploring alternative methods helped identify Copy Number Variations (CNVs) in a child with epilepsy.
Case Study
This case emphasizes the importance of considering different genetic tests for epilepsy, such as chromosomal microarray (CMA), epilepsy gene panels, and whole-exome sequencing, each with specific benefits and limitations.
Patient Profile:
A two-year-old male child born to a non-consanguineous couple presented with symptoms of febrile and non-febrile myoclonic seizures along with developmental delay. Normal development was observed until six months of age, after which neuroregression occurred. The patient experienced multiple febrile myoclonic seizures and one non-febrile myoclonic seizure, currently under anti-epileptic drug treatment.
Genetic Testing:
Whole exome sequencing was performed, and the reports seemed normal. The genetic tests most utilized in the evaluation of children with epilepsy include chromosomal microarray (CMA), epilepsy gene panels, and whole-exome sequencing (WES). Each test has its specific benefits and limitations. WES may identify genetic mutations in approximately 30% of patients with epilepsy. CMA may be abnormal in approximately 10% of individuals with epilepsy and is more likely to be abnormal in children with epilepsy and other conditions such as autism, developmental delay, and/or intellectual disability.
Genetic counselors at MapMyGenome further suggested CMA testing, which identified a heterozygous, pathogenic deletion on chromosome 2 at 2q24.3, associated with Migrating Epilepsy or Atypical Dravet Syndrome. The deleted genes included CSRNP3, GALNT3, TTC21B, SCN1A, SCN9A, SCN7A, and XIRP2.
Read more: Review all neurological examinations listed here
Clinical Implications:
The 2q24.3 microdeletion is a rare occurrence, presenting various phenotypes such as seizures, psychiatric diseases, developmental delay, learning difficulties, unusual facial characteristics, and cardiac involvement. The affected chromosome region encodes sodium channel genes linked to severe childhood epilepsy, including Dravet syndrome.
Parental testing, including chromosomal microarray and karyotype, was advised to rule out carrier status for the parents. Despite the rarity, future pregnancies were recommended to undergo CMA to identify potential chromosomal abnormalities.
While Next-Generation Sequencing (NGS) tests like clinical exome, whole exome, or gene panels are commonly used for epilepsy, this case underscores the importance of considering chromosomal microarray when panel NGS results are negative. CMA can reveal Copy Number Variation (CNV) changes associated with epilepsy, providing valuable insights into the genetic basis of the disorder.
Read full case study here: Case Study - Chromosomal Microarray in Epilepsy
This compelling case study from MapMyGenome illuminates the significance of genetic testing in the realm of neurological disorders. As we navigate the complexities of these conditions, genetic testing emerges not merely as a diagnostic method but as a beacon, guiding clinicians towards more accurate and personalized interventions, fostering a profound impact on patient outcomes and paving the way for advancements in neurological care.
MapmyGenome also offers comprehensive panels and various genetic testing options for neurological conditions. Our comprehensive neurology panel offers a targeted analysis of genes associated with major brain related disorders. By utilizing advanced sequencing technologies and variant analysis algorithms, we identify and interpret genetic variants within these genes. These variants provide valuable insights into the underlying molecular mechanisms of neurological conditions, helping to identify at-risk individuals, provide accurate diagnoses, predict disease progression, assess prognosis, and guide personalized treatment strategies.
References:
- https://www.who.int/news/item/27-02-2007-neurological-disorders-affect-millions-globally-who-report
- Salunkhe, Manish, et al. "Genetic testing in neurology: What every neurologist must know." Annals of Indian Academy of Neurology 25.3 (2022): 350.
