Medicamap: Pharmacogenetic Testing to Guide Cardiovascular Therapy
Aug 31, 2021
7301 Views
According to WHO, cardiovascular diseases (CVDs) are the most common cause of death around the world. In 2019, CVD contributed to over 17.9 million deaths, which is 32% of all global deaths. Of these, 85% were linked to heart attack and stroke. Low- and middle-income nations (which includes India) account for about three-quarters of CVD mortality (1).
Polypharmacy burden in India
In India, millions of people are on polypharmacy (taking more than four medications) to treat or prevent heart disease. Polypharmacy contributes to the risk of Adverse Drug Reactions (ADR), which could be a leading cause of morbidity, mortality, and readmission. The frequency of ADRs in the Indian population ranges from 1.7% to 25.1%, with 8% of them resulting in hospitalization; however, their reporting appears to be poor and inadequate (2). Currently, there are plenty of pharmacological measures available for preventing and treating cardiovascular diseases. However, the mechanism of treatment resistance and therapeutic failure is still unclear. Over the last decade, major pharmacogenetic advances have provided the impetus for using genetic information to guide therapy for cardiovascular patients to address toxicity and efficiency. (3)
Association between genetic variants and drug responses in cardiovascular diseases
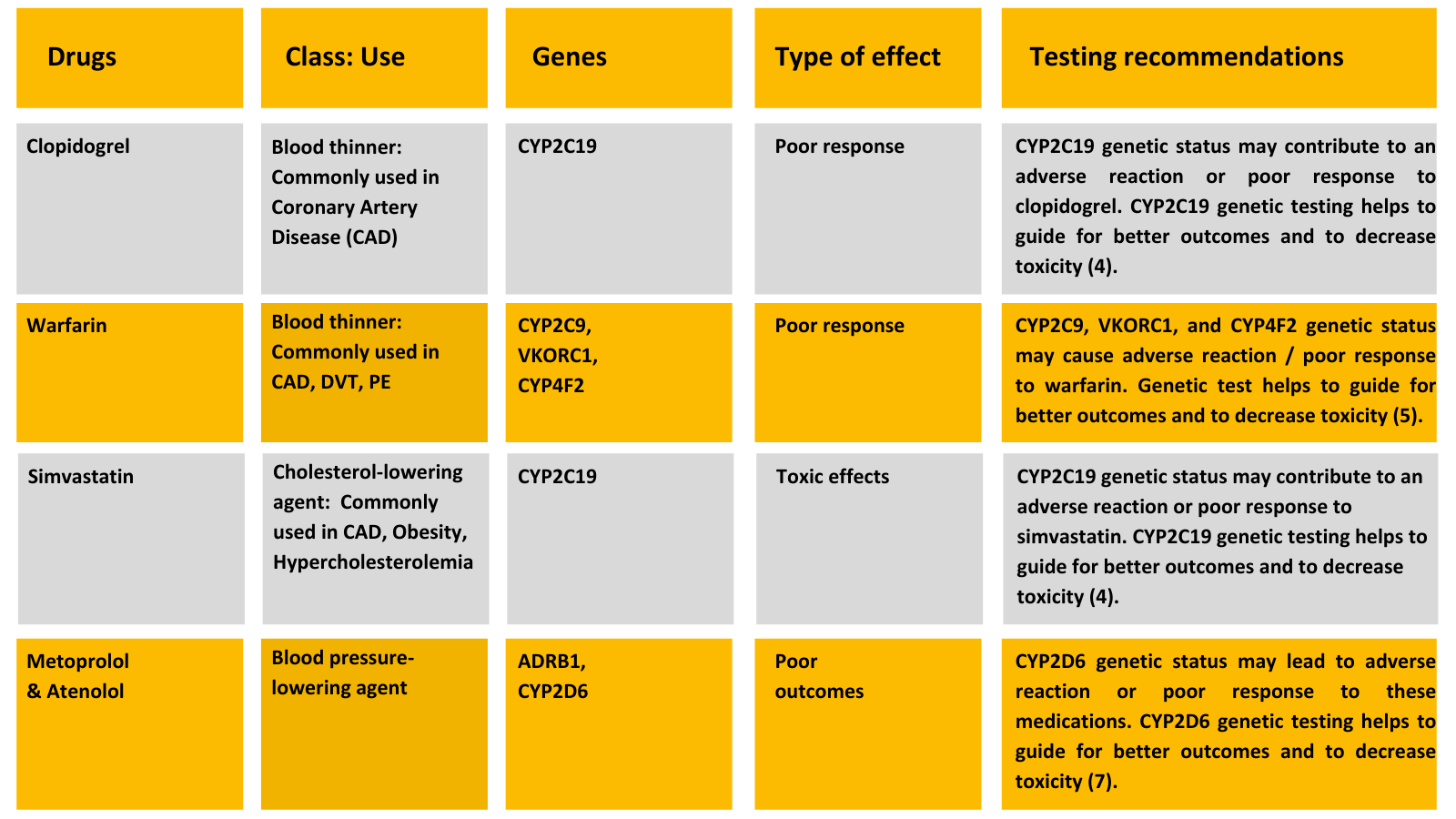
Heart disease medications are commonly metabolized in the liver by three enzymes: CYP2D6, CYP2C9, and CYP2C19. Other genetic pathways include CYP2C9, CYP3A4, CYP3A5, VKORC1, and SLCO1B1.
Medicamap
Medicamap is an advanced, next-generation comprehensive pharmacogenomic test to assess bio-markers to analyze an individual’s drug-response profile based on their genetic makeup.
Medicamap reports the highest clinically relevant genotype–drug associations for over a hundred drug compounds. The test has been developed for most of the common drugs used globally (including those prescribed to the Indian population) and covers all FDA-recommended pharmacogenomic specifications.
In addition to cardiovascular drugs, genetic markers for chemotherapeutic drug response and adverse reactions/toxicity, which have substantial evidence in the literature, have also been included in this test.
Medicamap – A patient safety tool
Statins (Cholesterol lowering agents) are commonly prescribed for coronary artery diseases and dyslipidemia and also to mitigate the risk of secondary complications such as heart failure and stroke. These drugs are well tolerated and have well-defined safety profiles. However, muscle discomfort and muscle pain is the most common and unpleasant side effect reported by patients. Several studies have found a strong link between drug-induced muscle injury and genetics (8 – 11).
“SLCO1B1 Genotyping has potential to predict and prevent direct statin-induced muscle damage, and to also prevent the increased incidence of CVD associated complications.”
Medicamap – A guide for better patient outcomes
Dual antiplatelet therapy (Aspirin & Clopidogrel) is effective in patients with coronary artery disease and percutaneous coronary intervention (PCI). However, some patients remain at risk for death, myocardial infarction (MI), and stent thrombosis due to various factors. One such factor could be patient genetics. In dual antiplatelet therapy, clopidogrel remains a mainstay for treating and preventing secondary complications of CVD. It is a prodrug that requires hepatic biotransformation to form an active metabolite in the liver by the enzyme CYP2C19. Research has linked the CYP2C19 genotype with clinical outcomes among clopidogrel-treated ACS patients, particularly those undergoing PCI (12,13).
Genetic variations in CYP2C19 are associated with reduced enzyme activity and decreased bio-activation of clopidogrel, leading to a subtherapeutic response. Testing for variants of the CYP2C19 gene could help tailor the treatment to achieve maximum benefits.
“Today, Almost every CAD patient is on clopidogrel; therefore, ordering a pharmacogenetic test which may help predict how you will respond to clopidogrel.”
References
- Cardiovascular diseases (CVDs). Fact sheet https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Sriram S, Ghasemi A, Ramasamy R, et al. Prevalence of adverse drug reactions at a private tertiary care hospital in south India. J Res Med Sci. 2011;16(1):16-25.
- Duarte JD, Cavallari LH. Pharmacogenetics to guide cardiovascular drug therapy. Nat Rev Cardiol. 2021 May 5. doi: 10.1038/s41569-021-00549-w. Epub ahead of print. PMID: 33953382.
- Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, Klein TE, Sabatine MS, Johnson JA, Shuldiner AR; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013 Sep;94(3):317-23. doi: 10.1038/clpt.2013.105. Epub 2013 May 22. PMID: 23698643; PMCID: PMC3748366.
- Johnson JA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SA, Lee MT, Gage BF, Kimmel SE, Perera MA, Anderson JL, Pirmohamed M, Klein TE, Limdi NA, Cavallari LH, Wadelius M. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin Pharmacol Ther. 2017 Sep;102(3):397-404. doi: 10.1002/cpt.668. Epub 2017 Apr 4. PMID: 28198005; PMCID: PMC5546947.
- Ramsey LB, Johnson SG, Caudle KE, Haidar CE, Voora D, Wilke RA, Maxwell WD, McLeod HL, Krauss RM, Roden DM, Feng Q, Cooper-DeHoff RM, Gong L, Klein TE, Wadelius M, Niemi M. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther. 2014 Oct;96(4):423-8. doi: 10.1038/clpt.2014.125. Epub 2014 Jun 11. PMID: 24918167; PMCID: PMC4169720.
- metoprolol – Overview, PharmGKB( Accessed on Aug 11)
- The role of SLCO1B1 genotyping in lowering cardiovascular risk, Charles A Brunette and Jason L Vassy, Pharmacogenomics 2021 22:11, 649-656
- Subashini C. Thambiah, Meor Fairuz Rizal Meor Anuar Shuhaili, Boon How Chew, Intan Nureslyna Samsudin, Hejar Abdul Rahman, Johnson Stanslas, Shariful Hasan & Zalinah Ahmad (2019) A pilot study on the association between SLCO1B1 RS4363657 polymorphism and muscle adverse events in adults with newly diagnosed dyslipidaemia who were prescribed a statin: the Malaysian primary health care cohort, Biomarkers, 24:7, 659-665, DOI: 10.1080/1354750X.2019.1648554
- Pharmacogenomics of statin-related myopathy: Meta-analysis of rare variants from whole-exome sequencing, Floyd JS, Bloch KM, Brody JA, Maroteau C, Siddiqui MK, et al. (2019) Pharmacogenomics of statin-related myopathy: Meta-analysis of rare variants from whole-exome sequencing. PLOS ONE 14(6): e0218115. https://doi.org/10.1371/journal.pone.0218115
- Pharmacogenetics and statin-related myopathy: what do we know?, Richard Myles Turner, Ivana Radman, Nada Bozina, and Ana Alfirevic, Pharmacogenomics 2020 21:12, 821-825.
- Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, Buonamici P, Antoniucci D, Abbate R, Gensini GF. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am J Cardiol. 2009 Mar 15;103(6):806-11. doi: 10.1016/j.amjcard.2008.11.048. Epub 2009 Jan 24. PMID: 19268736.
- Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, Bensimon G, Funck-Brentano C, Montalescot G. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009 Jan 24;373(9660):309-17. doi: 10.1016/S0140-6736(08)61845-0. Epub 2008 Dec 26. PMID: 19108880.